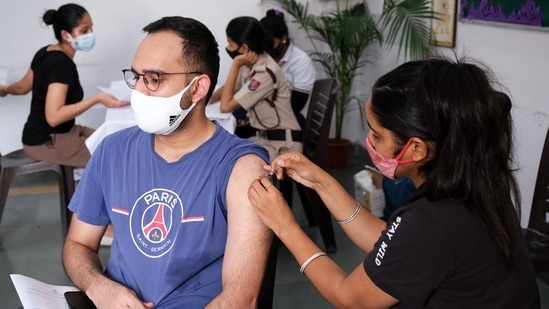
India opens gate for Moderna Covid-19 vaccine as Cipla gets DCGI nod
India on Tuesday made way for the fourth coronavirus vaccine in its drug basket after Mumbai-based pharmaceutical company Cipla got a nod from India's drug regulator DCGI to import the vaccine for restricted emergency use in the country, officials told HT.
India is already administering Oxford's Covishield, Bharat Biotech's Covaxin and Russia's Sputnik V in its fight against the deadly disease outbreak.
This comes a day after Cipla, on behalf of the US pharma major, requested the drug regulator for import and marketing authorisation of these jabs.
"Drugs Controller General of India (DCGI) has granted permission to Cipla to import Moderna's Covid-19 vaccine for restricted emergency use in the country as per the provisions of the New Drugs and Clinical Trial Rules , 2019 under Drugs and Cosmetics Act, 1940," a source told HT.
In separate communications, Moderna on June 27 informed DCGI that the US government has agreed to donate a certain number of doses of its Covid-19 vaccine through COVAX to India for use here and sought an approval from the Central Drugs Standard Control Organisation (CDSCO) for the vaccines.
Moderna's Covid-19 vaccine mRNA-1273, sold under the brand name 'Spikevax' in the United States, was developed by the pharmaceutical company in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA) in the US. It is composed of nucleoside-modified mRNA (modRNA) and encodes a SARS-CoV-2 spike protein encapsulated in lipid nanoparticles, according to the European Medicines Agency (EMA).
Earlier this month, Cipla had indicated its plan to fast-track approval to bring Moderna’s single-dose Covid-19 booster vaccine into India expeditiously, and that it has requested the government for indemnification and exemptions from price capping, bridging trials and basic customs duty. Cipla had not named the vaccine then.
Cipla was reportedly close to committing over $1 billion as advance to the US major for the import of close to 50 million doses, reports PTI.
India opens gate for Moderna Covid-19 vaccine as Cipla gets DCGI nod - Hindustan Times
Read More




No comments:
Post a Comment